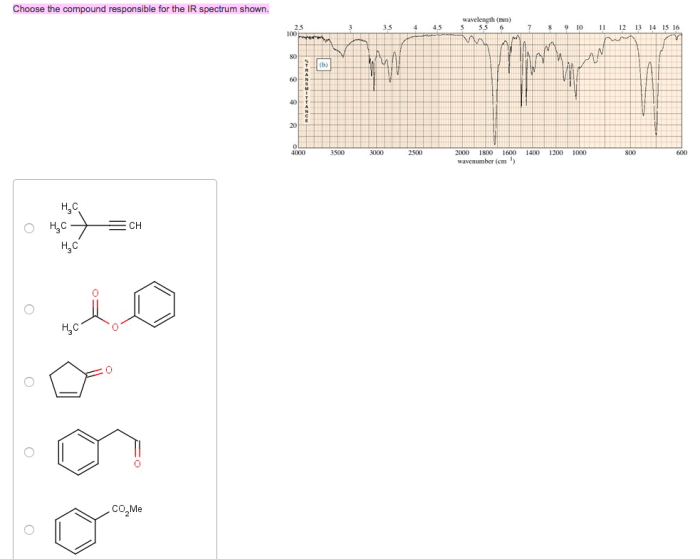
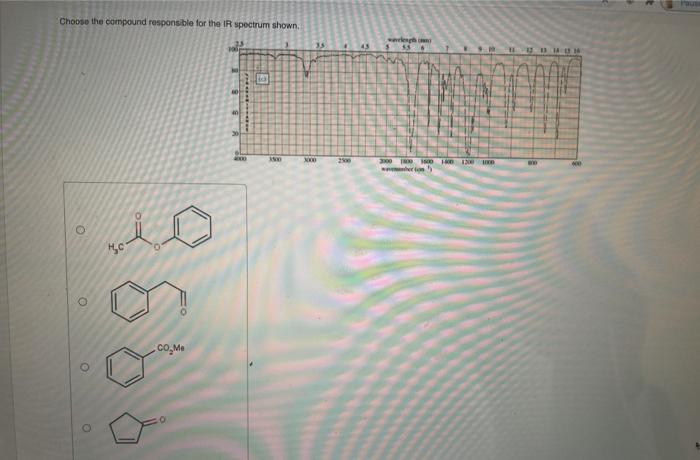
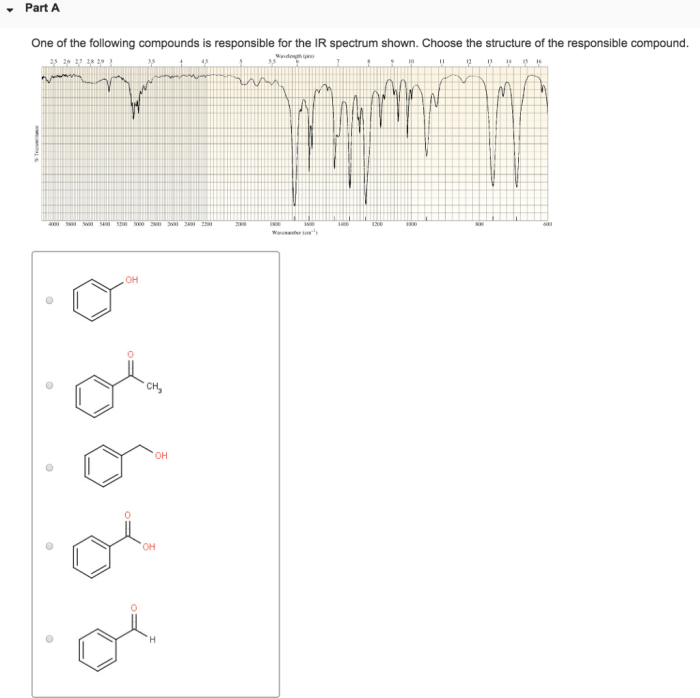
Choose the compound responsible for the ir spectrum shown. – Identify the Compound Behind the IR Spectrum: Unraveling Molecular Structures through Infrared Spectroscopy
Infrared (IR) spectroscopy unveils the secrets of molecular structures, providing a powerful tool for compound identification. This comprehensive guide delves into the intricacies of IR spectra, empowering readers to decipher the unique spectral signatures that reveal the chemical makeup of substances.
Introduction: Choose The Compound Responsible For The Ir Spectrum Shown.
Infrared (IR) spectroscopy is a powerful analytical technique used to identify compounds by analyzing the absorption of infrared radiation by the molecules. The IR spectrum of a compound provides information about its molecular structure, functional groups, and chemical bonding.
Key features of an IR spectrum include:
- Absorption bands:Peaks in the spectrum represent the absorption of IR radiation by specific functional groups.
- Wavenumber:The position of an absorption band on the x-axis is reported in wavenumbers (cm -1), which is inversely proportional to the wavelength of the absorbed radiation.
- Intensity:The height of an absorption band indicates the strength of the absorption and is related to the concentration of the functional group.
Identifying the Compound Responsible for the IR Spectrum
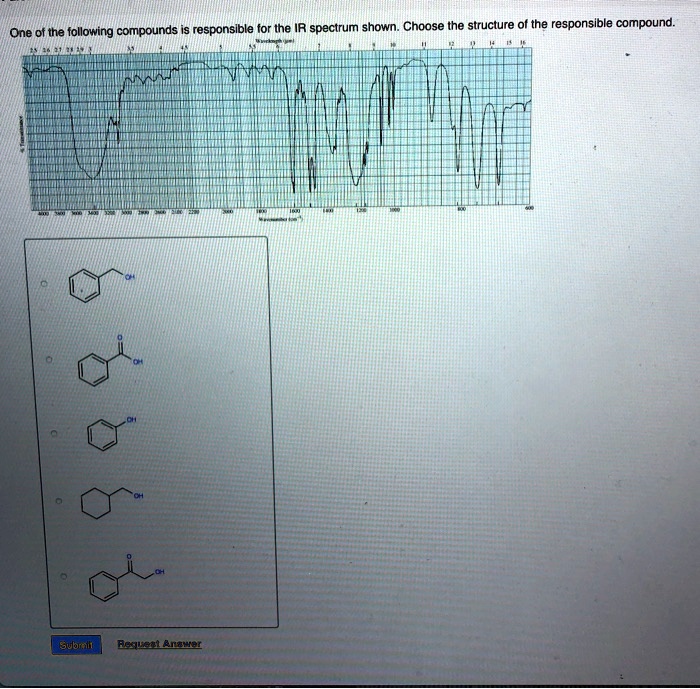
To identify the compound responsible for an IR spectrum, follow these steps:
- Identify the characteristic absorption bands present in the spectrum.
- Use a reference table or database to match the absorption bands to specific functional groups.
- Combine the information from the functional groups to determine the possible molecular structure of the compound.
- Compare the proposed structure to known compounds with similar IR spectra to confirm the identification.
Using IR Spectroscopy for Compound Identification
The following table lists common functional groups and their corresponding characteristic absorption bands:
| Functional Group | Characteristic Absorption Bands (cm-1) | Example Compounds |
|---|---|---|
| Alcohol (O-H) | 3200-3600 | Ethanol, methanol |
| Alkane (C-H) | 2850-3000 | Hexane, octane |
| Aldehyde (C=O) | 1660-1740 | Formaldehyde, acetaldehyde |
| Ketone (C=O) | 1680-1750 | Acetone, butanone |
| Carboxylic acid (O-H, C=O) | 2500-3000, 1680-1760 | Acetic acid, benzoic acid |
| Amine (N-H) | 3300-3500 | Ammonia, methylamine |
Advanced Techniques for IR Spectroscopy, Choose the compound responsible for the ir spectrum shown.
Advanced techniques such as Fourier transform infrared (FTIR) spectroscopy and attenuated total reflectance (ATR-FTIR) spectroscopy enhance the accuracy and sensitivity of IR spectroscopy for compound identification.
- FTIR spectroscopy:Uses a Fourier transform to convert the raw IR signal into a high-resolution spectrum, improving the signal-to-noise ratio and spectral resolution.
- ATR-FTIR spectroscopy:Allows IR analysis of solid or liquid samples without the need for sample preparation, making it a convenient and versatile technique.
Applications of IR Spectroscopy in Different Fields
IR spectroscopy is widely used in various fields, including:
- Chemistry:Identifying organic and inorganic compounds, determining molecular structure, and studying reaction mechanisms.
- Biochemistry:Analyzing proteins, nucleic acids, and other biological molecules, and studying their structure and function.
- Pharmaceutical analysis:Identifying and characterizing active pharmaceutical ingredients, ensuring drug purity and quality.
- Environmental monitoring:Detecting and identifying pollutants in air, water, and soil samples.
Common Queries
What is the significance of IR spectroscopy in chemistry?
IR spectroscopy provides crucial information about the functional groups present in a compound, enabling the identification and characterization of organic and inorganic substances.
How can I interpret the absorption bands in an IR spectrum?
Each functional group exhibits characteristic absorption bands in an IR spectrum, which correspond to specific vibrational modes of the molecule. By matching these bands to reference data, the presence of specific functional groups can be determined.
What are the limitations of IR spectroscopy?
IR spectroscopy may not be suitable for highly symmetrical molecules or compounds with weak or no dipole moments, as these may result in weak or absent absorption bands.