Al2O3 check all that apply. dispersion ionic dipole-dipole h-bonding: Delving into the diverse forces that shape the nature of Al2O3, this exploration unveils the interplay of dispersion, ionic, dipole-dipole, and hydrogen bonding interactions, providing a comprehensive understanding of their influence on the material’s properties and behavior.
Dispersion forces, arising from the instantaneous polarization of molecules, contribute to the cohesive nature of Al2O3. Ionic bonding, involving the electrostatic attraction between oppositely charged ions, plays a significant role in determining the crystal structure and stability of the material.
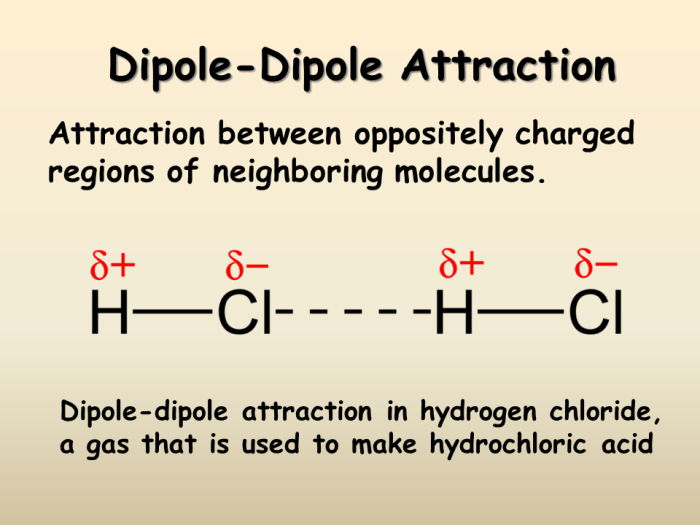
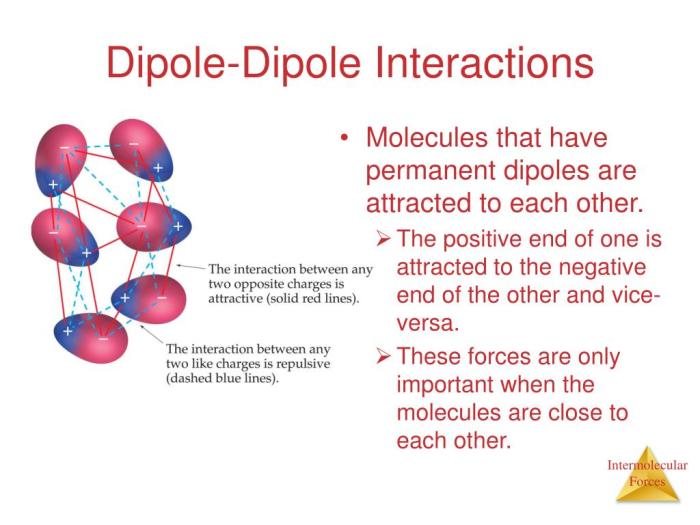
Dipole-dipole interactions, resulting from the alignment of polar molecules, further enhance the cohesive forces within Al2O3. Hydrogen bonding, characterized by the attraction between a hydrogen atom and an electronegative atom, also contributes to the structural integrity and properties of the material.
Intermolecular Forces in Al2O3: Al2o3 Check All That Apply. Dispersion Ionic Dipole-dipole H-bonding
Al2O3 is a versatile material with applications in various fields. Understanding the intermolecular forces that govern its behavior is crucial for optimizing its properties and performance. This article delves into the different types of intermolecular forces present in Al2O3, including dispersion forces, ionic bonding, dipole-dipole interactions, and hydrogen bonding.
Dispersion Forces
Dispersion forces arise from the temporary fluctuations in electron distribution, creating instantaneous dipoles. In Al2O3, these fluctuations occur due to the movement of electrons within the covalent bonds. The resulting dipoles interact with each other, leading to attractive forces between molecules.
Dispersion forces are relatively weak compared to other intermolecular forces but contribute to the overall cohesive properties of Al2O3.
Ionic Bonding, Al2o3 check all that apply. dispersion ionic dipole-dipole h-bonding
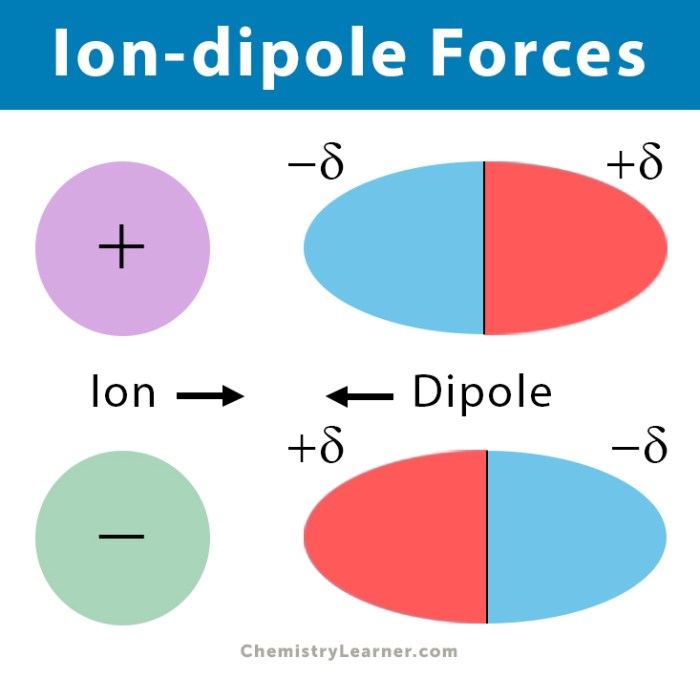
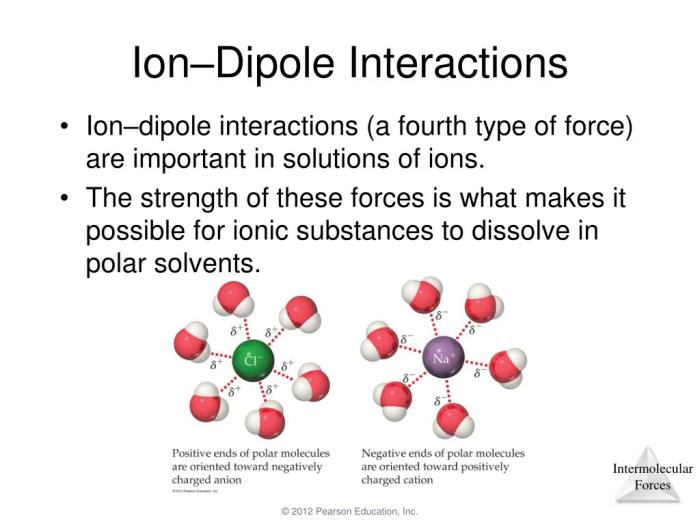
Al2O3 is primarily characterized by ionic bonding, where aluminum atoms donate electrons to oxygen atoms, forming positively charged aluminum ions (Al3+) and negatively charged oxygen ions (O2-). The electrostatic attraction between these ions holds the crystal structure of Al2O3 together.
Ionic bonding imparts strength, rigidity, and high melting and boiling points to Al2O3.
Dipole-Dipole Interactions
Dipole-dipole interactions occur between molecules that possess permanent dipoles. In Al2O3, the oxygen atoms have a partial negative charge, while the aluminum atoms have a partial positive charge, creating a permanent dipole moment. These dipoles interact with each other, resulting in attractive forces between molecules.
Dipole-dipole interactions contribute to the polar nature of Al2O3 and influence its solubility and reactivity.
Hydrogen Bonding
Hydrogen bonding is a special type of dipole-dipole interaction that occurs when a hydrogen atom is covalently bonded to a highly electronegative atom, such as oxygen or nitrogen. In Al2O3, hydrogen bonding can occur between the hydrogen atoms of hydroxyl groups (OH-) and the oxygen atoms of the Al-O bonds.
Hydrogen bonding strengthens the intermolecular interactions, leading to higher melting and boiling points, and influencing the material’s mechanical properties.
Essential FAQs
What are the key factors influencing the properties of Al2O3?
The properties of Al2O3 are primarily governed by the interplay of dispersion, ionic, dipole-dipole, and hydrogen bonding interactions.
How does dispersion force contribute to the behavior of Al2O3?
Dispersion forces, arising from the instantaneous polarization of molecules, contribute to the cohesive nature and physical properties of Al2O3.
What role does ionic bonding play in shaping the structure of Al2O3?
Ionic bonding, involving the electrostatic attraction between oppositely charged ions, plays a significant role in determining the crystal structure and stability of Al2O3.