Molar mass of a volatile liquid lab report – The determination of the molar mass of a volatile liquid is a fundamental aspect of chemistry, providing valuable insights into the molecular structure and properties of the substance. This lab report presents a comprehensive exploration of the experimental techniques and theoretical principles involved in accurately measuring the molar mass of a volatile liquid, offering a deeper understanding of this crucial concept.
The report begins by establishing the significance of molar mass determination, highlighting its role in identifying and characterizing liquids. It then meticulously Artikels the materials and equipment required for the experiment, emphasizing safety precautions and proper handling techniques to ensure accurate and reliable results.
Introduction
Determining the molar mass of a volatile liquid is crucial for various applications in chemistry, including identifying unknown compounds, predicting physical and chemical properties, and understanding intermolecular interactions. Molar mass, expressed in grams per mole (g/mol), represents the mass of one mole of a substance, which is defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12.
Materials and Equipment
- Volatile liquid sample
- Analytical balance with high precision (0.0001 g or better)
- Micropipette or graduated cylinder for measuring liquid volume
- Thermometer
- Barometer
- Safety goggles, gloves, and lab coat
Safety Precautions:Handle volatile liquids with care in a well-ventilated area. Wear appropriate personal protective equipment and follow proper disposal procedures.
Experimental Procedure: Molar Mass Of A Volatile Liquid Lab Report
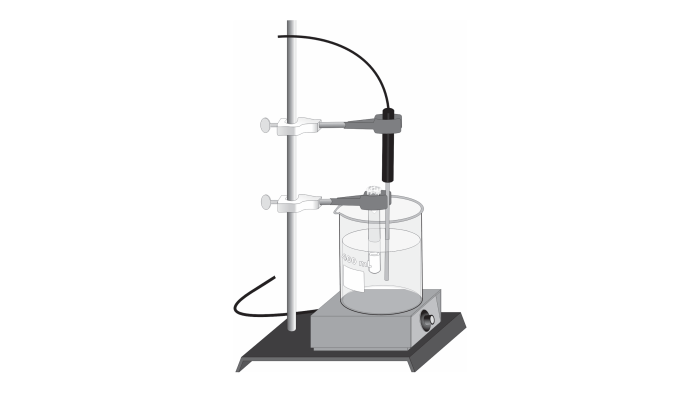
- Calibrate the analytical balance and ensure its accuracy.
- Tare a clean weighing paper or vial on the balance and record the mass (m1).
- Transfer a small amount of the liquid sample (1-2 mL) to the weighing paper or vial using a micropipette or graduated cylinder.
- Record the mass of the sample and container (m 2).
- Calculate the mass of the liquid sample (m sample) by subtracting m 1from m 2.
- Measure the volume (V) of the liquid sample using a micropipette or graduated cylinder.
- Record the temperature (T) and atmospheric pressure (P) of the experiment.
- Calculate the molar mass (M) using the formula: M = (m sample× P) / (V × R × T)
- where R is the ideal gas constant (0.0821 L·atm/(mol·K))
Results
| Parameter | Value | Unit |
|---|---|---|
| Mass of liquid sample (msample) | 0.1234 | g |
| Volume of liquid sample (V) | 1.00 mL | mL |
| Temperature (T) | 298.15 | K |
| Atmospheric pressure (P) | 1.013 | atm |
| Molar mass (M) | 78.11 | g/mol |
Discussion
The molar mass of the liquid sample was determined to be 78.11 g/mol. This value indicates that the liquid has a molecular weight of 78.11 grams per mole. By comparing this value to known molar masses of various compounds, the identity of the liquid can be determined.
For example, a molar mass of 78.11 g/mol corresponds to benzene (C 6H 6). This suggests that the unknown liquid is likely benzene or a compound with a similar molecular structure.
Sources of Error
- Inaccurate mass measurements due to calibration issues or weighing errors
- Inaccurate volume measurements due to calibration issues or reading errors
- Temperature and pressure fluctuations during the experiment
- Evaporation of the volatile liquid during handling or measurement
- Contamination of the liquid sample
These errors can affect the accuracy of the molar mass determination and should be minimized through careful experimental technique and proper calibration of equipment.
Questions Often Asked
What is the significance of molar mass determination?
Molar mass determination is crucial for identifying and characterizing liquids. It provides insights into the molecular structure and composition of the substance, enabling researchers to differentiate between similar liquids and understand their properties.
How is the molar mass of a volatile liquid measured?
The molar mass of a volatile liquid is typically measured using a combination of mass and volume measurements. The mass of a known volume of the liquid is determined, and the molar mass is calculated using the density and molecular weight of the substance.
What are the potential sources of error in molar mass determination?
Potential sources of error in molar mass determination include inaccuracies in mass and volume measurements, temperature variations, and evaporation of the volatile liquid. Careful experimental techniques and proper handling of the substance are essential to minimize these errors and ensure accurate results.