Embark on an enlightening journey into the fundamental building blocks of matter with our comprehensive guide, “Parts of the Atom Worksheet Answers.” This authoritative resource unravels the intricate structure of atoms, empowering you with a profound understanding of their essential components.
Delve into the nucleus, the electron cloud, and the energy levels that govern the behavior of electrons. Our meticulously crafted worksheet answers provide a structured and accessible approach to mastering this foundational concept.
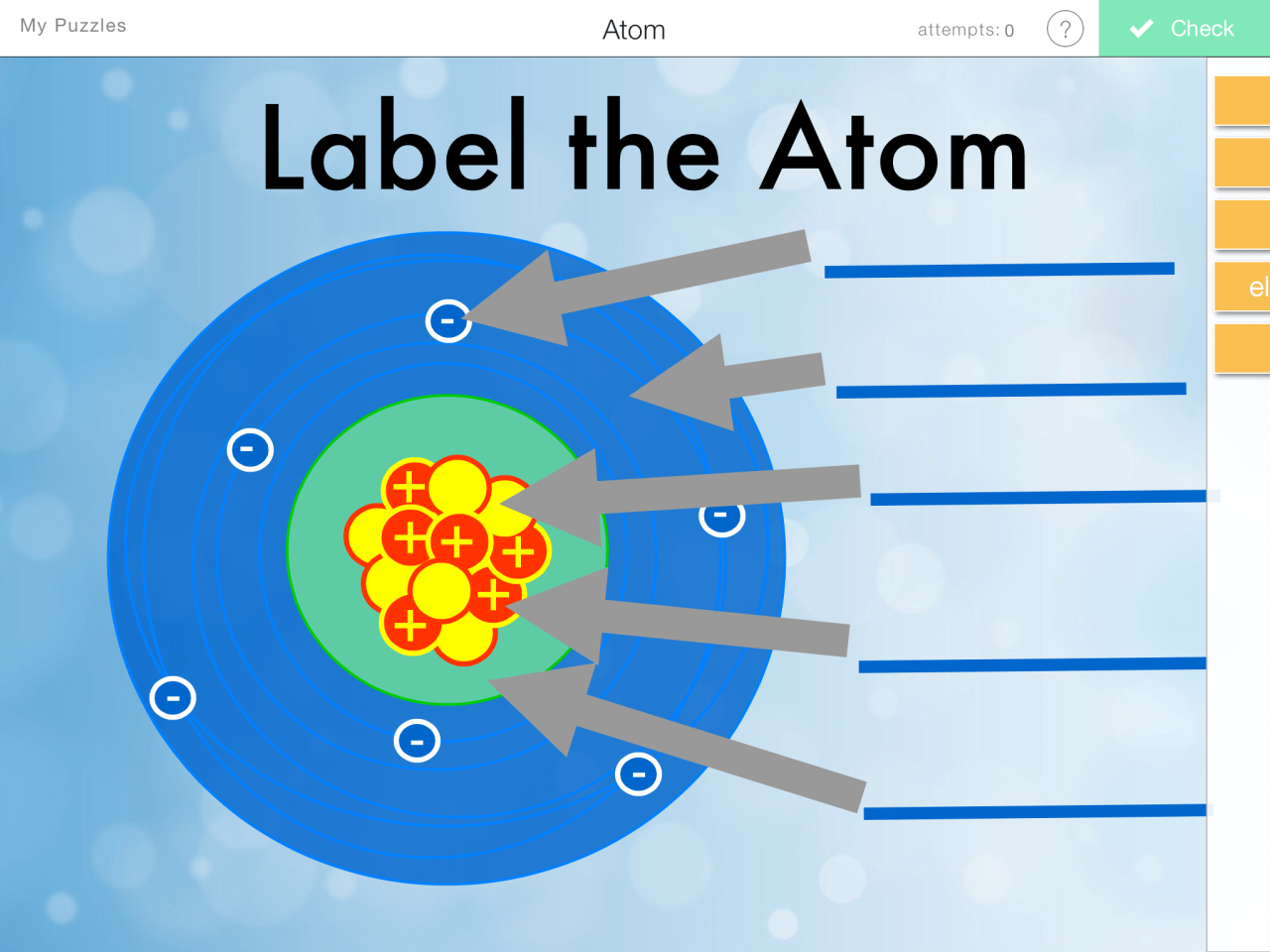
Parts of an Atom
Atoms, the fundamental building blocks of matter, are composed of three fundamental particles: protons, neutrons, and electrons. These particles vary in their charge, mass, and location within the atom.
| Particle | Symbol | Charge | Location |
|---|---|---|---|
| Proton | p+ | +1 | Nucleus |
| Neutron | n0 | 0 | Nucleus |
| Electron | e- | -1 | Electron cloud |
Protons and neutrons reside in the atom’s nucleus, a dense, positively charged core. Electrons, on the other hand, occupy the electron cloud, a region surrounding the nucleus. The arrangement of these particles determines the atom’s properties and behavior.
Atomic Structure: Parts Of The Atom Worksheet Answers
The nucleus of an atom contains protons and neutrons, collectively known as nucleons. Protons contribute a positive charge, while neutrons are electrically neutral. The number of protons in the nucleus defines the element to which the atom belongs.Surrounding the nucleus is the electron cloud, a region where electrons are found.
Electrons occupy specific energy levels, which are determined by their distance from the nucleus. Each energy level consists of subshells, which are characterized by different shapes and orientations. Electrons within these subshells can transition between energy levels by absorbing or emitting energy in the form of photons.
Worksheet Answers
| Question | Answer |
|---|---|
| What is the symbol for a proton? | p+ |
| Where are electrons located in an atom? | Electron cloud |
| What is the charge of a neutron? | 0 |
| How many protons are in the nucleus of a carbon atom? | 6 |
| What is the energy level closest to the nucleus? | 1s |
Applications and Examples
Understanding the parts of an atom has far-reaching applications in various fields. In chemistry, it helps explain chemical bonding, reactivity, and the properties of different elements. In physics, it provides the foundation for understanding nuclear reactions, radiation, and the behavior of matter at the atomic and subatomic levels.For
instance, in nuclear medicine, the decay of radioactive isotopes is used for diagnostic imaging and cancer treatment. In materials science, manipulating the arrangement of atoms can lead to the development of novel materials with enhanced properties. Additionally, atomic structure plays a crucial role in fields such as astrophysics, quantum computing, and nanotechnology.
Question Bank
What are the fundamental particles that make up an atom?
Protons, neutrons, and electrons
Where are protons and neutrons located within an atom?
Nucleus
What is the electron cloud?
A region of space around the nucleus where electrons are likely to be found
What determines the energy level of an electron?
The distance from the nucleus
